6. CLB and IgG subclasses
6.1 Kits and reagents
The CLB was among the first manufacturers of blood group and immune reagents. The institute has a long and successful record in biomedical research, experimental and clinical immunology and transfusion medicine. By virtue of its research facilities and diagnostic laboratories, CLB has been able to develop a broad range of products, including several innovative reagents for diagnostic use and for fundamental and clinical research. Since the 1970's CLB manufactures high-quality kits, reagents, standards and controls for quantification of human IgG subclasses.
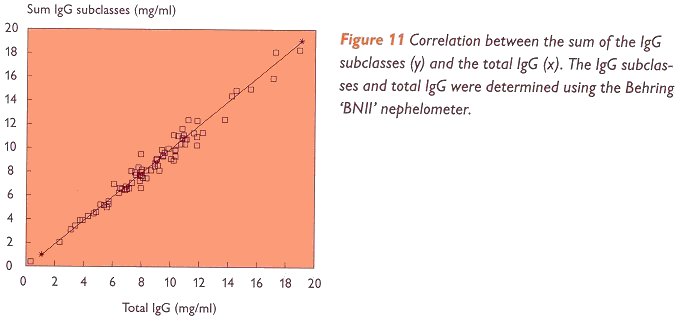
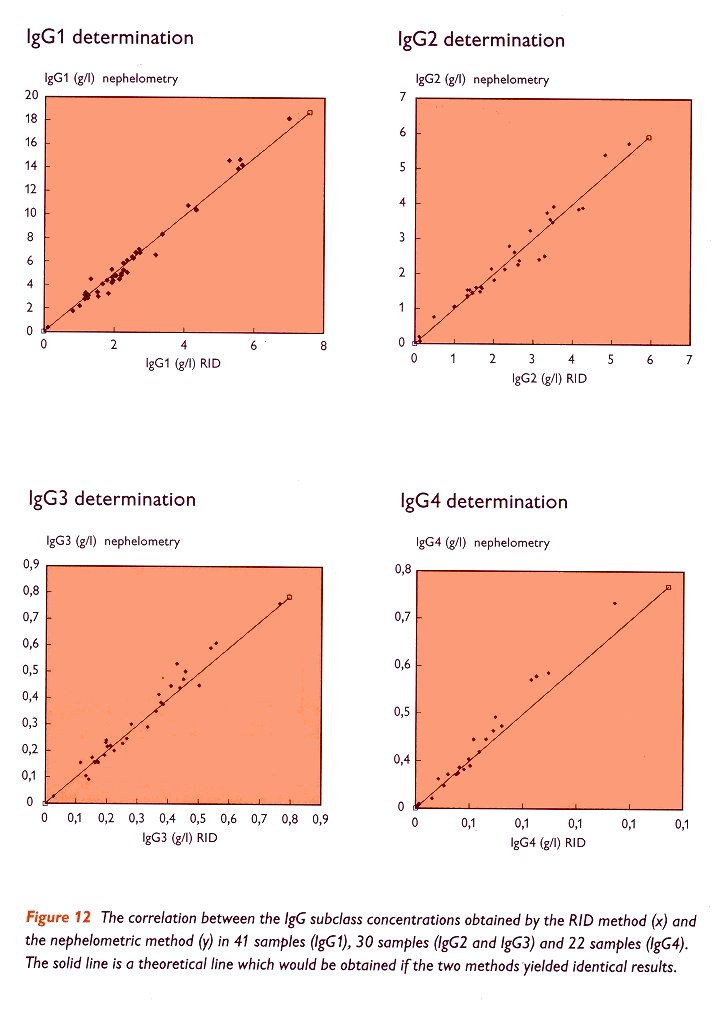
It has always been one of CLB's principles that a value obtained for the IgG subclass level in one serum sample should be the same, regardless of the technique used. For one thing, this has resulted in a set of reference values (table III). This unique set of reference values can only be used if a high correlation exists between the results obtained by different techniques. It is seen that the results obtained by different IgG subclass kits from CLB show a good correlation. In figure 10 the good correlation. In figure 10 the good correlation of the CLB nephelometric 'Array' kit and the CLB RIA kit is shown.

6.2 CLB Quality Survey Service for IgG subclass assays
As a service to its customers, the CLB Department of Reagents provides a free Quality Survey for in-house assessment of local human IgG subclass assays. Laboratories in any part of the world may participate.
Twice a year, 3 encoded samples (normal, as well as patient sera) are distributed among the participating laboratories. Levels of IgG1, IgG2, IgG3, IgG4 and total IgG are determined by the respective laboratories and all results are evaluated at CLB. This procedure is suitable to detect any systematic errors in the assays performed by the participating laboratories.
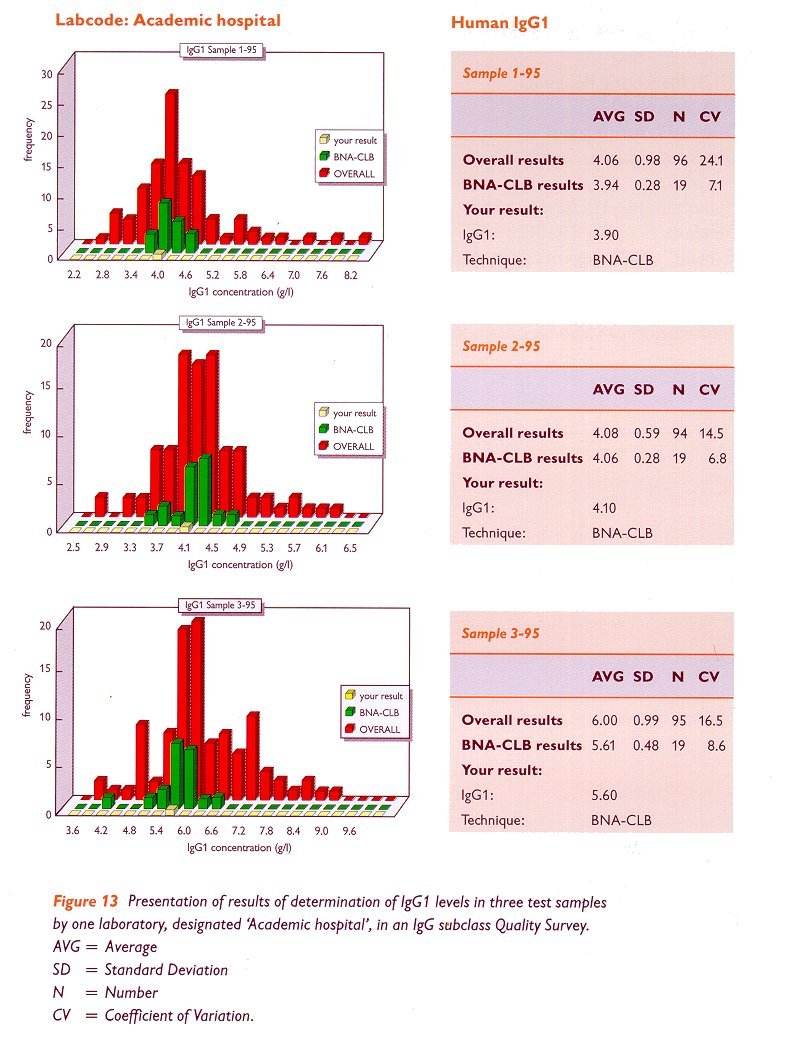
In figure 11 we show an example of some representative data from one of our 1995 Quality Surveys. The participating laboratories quantified the four IgG subclasses and total IgG in the three samples, by means of RIA, ELISA, nephelometry, turbidimetry, affinity chromatography or other techniques with kits or reagents supplied by the CLB or by other manufacturers. The example given in figure 11 shows the results of IgG1 determination on three different samples, which were obtained by one of the laboratories (designated here as 'TEST'), using the CLB-BNA (Behring nephelometer) kit. These results are compared with those from other participating laboratories using the same technique and reagents, as well as with the overall results, obtained by all techniques used. The data are presented in the format of histograms. By regularly participating in such Quality Surveys, laboratories will be able to check the reliability of their tests.