Previous page Next page
2 IgG subclasses and humoral immunity
2.1 Immunoglobulins and humoral immunity
The glycoprotein immunoglobulin G (IgG), a major effector molecule of the humoral immune response in man, accounts for about 75% of the total immunoglobulins in plasma of healthy individuals. The immunoglobulins of the other four classes, IgM, IgA, IgD and IgE, each of which has characteristic properties and functions, constitute the other 25% of the immunoglobulins (3). Antibodies of the IgG class express their predominant activity during a secondary antibody response. Thus, the appearance of specific IgG antibodies generally corresponds with the 'maturation' of the antibody response, which is switched on upon repeated contact with an antigen. In comparison to antibodies of the IgM class, IgG antibodies have a relatively high affinity and persist in the circulation for a long time. The five classes of human immunoglobulins can be distinguished on the basis of the amino acid composition. This is also the basis for antigenic differences between these molecules and for immunological recognition by specific antisera/antibodies (4).
2.2 Molecular basis of immunoglobulin synthesis
The polypeptide chains of immunoglobulins are encoded by three non-linked cluster of autosomal genes, one cluster coding for heavy chains of all classes and subclasses, a second one for kappa-light chains and a third one for lambda light chains. These three gene clusters are called the H-, k-and y gene families respectively. In humans the H gene family is on chromosome 14, the k gene family is on chromosome 2 and the y gene family is on chromosome 22. Molecular genetic studies have revealed the arrangement of gene segments within the heavy chain and light chain families. Each heavy chain is encoded by 4 distinct types of gene segments, designated VH (variable), D (diversity), JH (joining) and CH(constant). The variable region of the heavy chain is encoded by the VH, D and JH segments. The light chains are encoded by the 3 gene segments, VL, JL and CL. The variable region of the light chains is encoded by the VL and JL segments.
The C gene segments of the heavy and light chains encode for the constant regions. Nine immunoglobulin heavy chain isotypes are found in humans: IgM, IgG, IgE, IgG (isotypically comprising four different subclasses IgG1, IgG2, IgG3 and IgG4) and IgA (with subclasses IgA1 and IgA2).
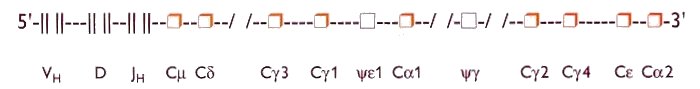
The CH gene segments determine the class and/or subclass of the heavy chain, whereas the VH, D and JH regions determine the antigen-recognizing part of the immunoglobulin molecule. The heavy chain constant region genes lie 3' to the VH, D and JH genes. During the maturation of progenitor B cells to mature B cells an active heavy chain exon is formed by VH, D, JH integration (recombined VHDJH gene), followed by linkage to a certain CH-gene locus. This matrix is transcribed to mRNA and subsequently translated to an immunoglobulin heavy chain molecule The CH gene closest to the JH locus, the Cm gene (IgM), is the first isotype gene to be expressed. The other CH genes can subsequently be expressed by 'downstream' switching mechanisms with simultaneous deletion of the original isotypic CH genes. According to this model the order of the C region genes on the chromosome largely determines the immunoglobulin isotype in an immune response. However, this mechanism may be influenced by isotype-specific switch factors such as IL-4 and IL-5. The relative localisation of the genes controlling the isotypes of the immunoglobulin classes and subclasses has the following 5' to 3'-oriented sequence on the DNA of chromosome 14 (figure 3):
Cm (IgM) - Cd (IgD) - Cg3 (IgG3) - Cg1(IgG1)-pseudogene Ce1 - Ca1 (IgA1) -pseudogene Cg - Cg2 (IgG2) - Cg4 (IgG4) - Ce (IgE) - Ca2 (IgA2).
By comparing the four IgG subclass genes, it becomes clear that the genes for IgG3 and IgG1 are close together, as are those for IgG2 and IgG4(5). The synthesis of each subclass is independently regulated. IgG1 and IgG3 levels generally rise more quickly than those of IgG2 and IgG4, possibly reflecting the occurrence of a successive 'downstream switch' in the immunoglobulin heavy chain constant region genes(6).
|
|
|
Figure 3. Schematic representation of the arrangement on chromosome 14 of the gene loci governing the constant region of the human immunoglobulin heavy chain. VH denotes variable gene segments, D the diversity gene segments, JH the joining gene segments.
Cm denotes IgM-CH gene, Cd the IgD-CH gene, Cg3 the IgG3-CH gene, Cg1 the IgG1-CH gene, the y
e
1 a pseudo gene (not expressed). Ca 1 the IgA1-CH gene, y g a pseudo gene (not expressed), Cg 2 the IgG2-CH gene, Cg 4 the IgG4-CH gene, Ce the IgE-CH gene. Ca 2 the IgA2-CH gene. |
Gene deletions resulting in a complete deficiency, a total lack, of one or more IgG subclasses are very rare(7). In fact, most abnormalities are based upon regulatory defects, resulting in a decreased level (deficiency) rather than a total lack of one or more immunoglobulin (sub)classes. In some patients, an IgG2 deficiency is associated with a deficiency of IgG4, IgA1 and IgA2. Indeed, the genes encoding IgG2, IgG4 and the IgA subclasses are closely linked and this combined deficiency is due to a regulation defect of the 'downstream switch' in the heavy chain loci. This may result in a Maturation arrest in the immune response. It is likely that the expression of the immunoglobulins whose genes are located downstream in the CH region (especially IgG4 and IgE) requires more help from T helper 2 cells in comparison with upstream isotypes (IgG1 and IgG3) (8,9,10). IgG4 and also IgG2 deficiencies are found in imunodeficiency states characterised by a predominant T cell defect, such as in ataxia telangiectasia, AIDS and immune reconstitution after bone marrow transplantation (see section 4).
The observation that IgG subclass-limited responses occur suggests that the repertoire of V genes, as expressed in antibody diversity, differs between some subclasses.
Previous page Next page
